Page 403 - Mechanism and Theory in Organic Chemistry
P. 403
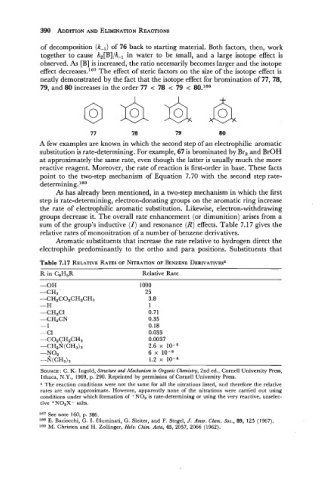
of decomposition (k-l) of 76 back to starting material. Both factors, then, work
together to cause k,[B]/k-I in water to be small, and a large isotope effect is
observed. As [B] is increased, the ratio necessarily becomes larger and the isotope
effect decreases.l6I The effect of steric factors on the size of the isotope effect is
neatly demonstrated by the fact that the isotope effect for bromination of 77, 78,
79, and 80 increases in the order 77 < 78 < 79 < 80.1s8
A few examples are known in which the second step of an electrophilic aromatic
substitution is rate-determining. For example, 67 is brominated by Br, and BrOH
at approximately the same rate, even though the latter is usually much the more
reactive reagent. Moreover, the rate of reaction is first-order in base. These facts
point to the two-step mechanism ~f Equation 7.70 with the second step rate-
determining.lsg
As has already been mentioned, in a two-step mechanism in which the first
step is rate-determining, electron-donating groups on the aromatic ring increase
the rate of electrophilic aromatic substitution. Likewise, electron-withdrawing
groups decrease it. The overall rate enhancement (or dimunition) arises from a
sum of the group's inductive (I) and resonance (R) effects. Table 7.17 gives the
relative rates of mononitration of a number of benzene derivatives.
Aromatic substituents that increase the rate relative to hydrogen direct the
electrophile predominantly to the ortho and para positions. Substituents that
Table 7.17 RELATIVE RATES OF NITRATION BENZENE DERIVATIVES~
OF
R in C6H,R Relative Rate
SOURCE: C. K. Ingold, Structure and Mechanism in Organic Chemistry, 2nd ed., Cornell University Prcss,
Ithaca, N.Y., 1969, p. 290. Reprinted by permission of Cornell University Press.
a The reaction conditions were not the same for all the nitrations listed, and therefore the relative
rates are only approximate. However, apparently none of the nitrations were carried out using
conditions under which formation of +NO2 is rate-determining or using the very reactive, unselec-
tive +N02X- salts.
16' See note 160, p. 386.
E. Baciocchi, G. I. Illuminati, G. Sleiter, and F. Stegel, J. Amer. Chenr. SOC., 89, 125 (1967).
168 M. Christen and H. Zollinger, Helv. Chim. Acta, 45, 2057, 2066 (1962).