Page 401 - Mechanism and Theory in Organic Chemistry
P. 401
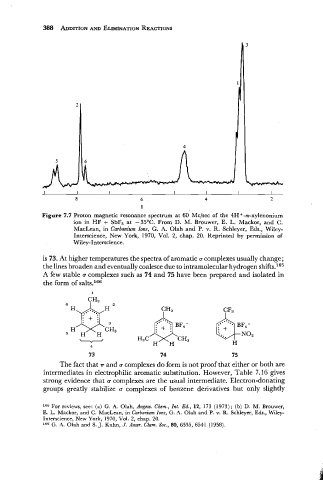
Figure 7.7 Proton magnetic resonance spectrum at 60 Mclsec of the 4H+-m-xylenonium
ion in HF + SbF, at -35OC. From D. M. Brouwer, E. L. Mackor, and C.
MacLean, in Carbonium Ions, G. A. Olah and P. v. R. Schleyer, Eds., Wiley-
Interscience, New York, 1970, Vol. 2, chap. 20. Reprinted by permission of
Wiley-Interscience.
is 73. At higher temperatures the spectra of aromatic o complexes usually change;
the lines broaden and eventually coalesce due to intramolecular hydrogen shifts.165
A few stable o complexes such as 74 and 75 have been prepared and isolated in
the form of salts.16=
The fact that .rr and a complexes do form is not proof that either or both are
intermediates in electrophilic aromatic substitution. However, Table 7.16 gives
strong evidence that o complexes are the usual intermediate. Electron-donating
groups greatly stabilize o complexes of benzene derivatives but only slightly
la5 For reviews, see: (a) G. A. Olah, Angew. Chem., Int. Ed., 12, 173 (1973); (b) D. M. Brouwer,
E. L. Mackor, and C. MacLean, in Carbonium Ions, G. A. Olah and P. v. R. Schleyer, Eds., Wiley-
Interscience, New York, 1970, Vol. 2, chap. 20.
lea G. A. Olah and S. J. Kuhn, J. Amer. Chem. Soc., 80, 6535, 6541 (1958).