Page 397 - Mechanism and Theory in Organic Chemistry
P. 397
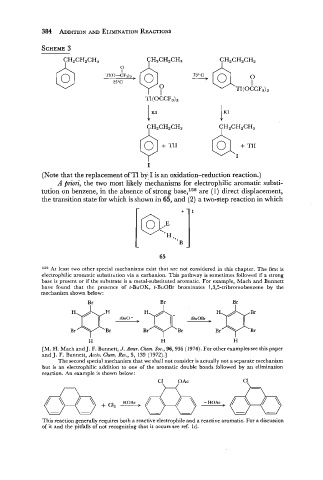
(Note that the replacement of Tl by I is an oxidation-reduction reaction.)
A priori, the two most likely mechanisms for electrophilic aromatic substi-
tution on benzene, in the absence of strong base,156 are (1) direct displacement,
the transition state for which is shown in 65, and (2) a two-step reaction in which
15= At least two other special mechanisms exist that are not considered in this chapter. The first is
electrophilic aromatic substitution via a carbanion. This pathway is sometimes followed if a strong
base is present or if the substrate is a metal-substituted aromatic. For example, Mach and Bunnett
have found that the presence of t-BuOK, t-BuOBr brominates 1,3,5-tribromobenzene by the
mechanism shown below:
[M. H. Mach and J. F. Bunnett, J. Amer. Chem. Soc., 96,936 (1974). For other examples see this paper
and J. F. Bunnett, Accts. Chem. Res., 5, 139 (1972).]
The second special mechanism that we shall not consider is actually not a separate mechanism
but is an electrophilic addition to one of the aromatic double bonds followed by an elimination
reaction. An example is shown below:
This reaction generally requires both a reactive electrophile and a reactive aromatic. For a discussion
of it and the pitfalls of not recognizing that it occurs see ref. Id.