Page 402 - Mechanism and Theory in Organic Chemistry
P. 402
Electrophilic Aromatic Substitution 389
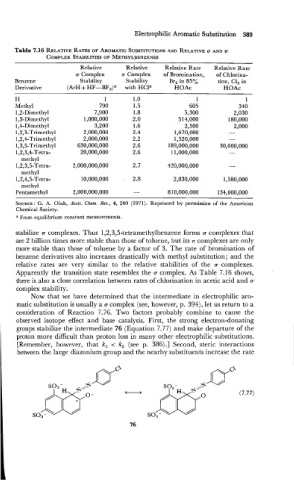
Table 7.16 RELATIVE RATES OF AROMATIC SUBSTITUTIONS ~LATIVE u AND n
AND
COMPLEX STABILITIES METHYLBENZENES
OF
Relative Relative Relative Rate Relative Rate
u Complex rr Complex of Bromination, of Chlorina-
Benzene Stability Stability Br, in 85% tion, C1, in
Derivative (ArH + HF-BF,)' with HCla HOAc HOAc
H 1 1 .o 1 1
Methyl 790 1.5 605 340
1,2-Dimethyl 7,900 1.8 5,300 2,030
1,3-Dimethyl 1,000,000 2.0 5 14,000 180,000
1,4-Dimethyl 3,200 1.6 2,500 2,000
1,2,3-Trimethyl 2,000,000 2.4 1,670,000 -
1,2,4-Trimethyl 2,000,000 2.2 1,520,000 -
1,3,5-Trimethyl 630,000,000 2.6 189,000,000 30,000,000
1,2,3,4-Tetra- 20,000,000 2.6 1 1,000,000 -
methyl
1,2,3,5-Tetra- 2,000,000,000 2.7 420,000,000 -
methyl
1,2,4,5-Tetra- 10,000,000 , 2.8 2,830,000 1,580,000
methyl
Pentamethyl 2,000,000,000 - 8 10,000,000 134,000,000
SOURCE: G. A. Olah, Accts. Chem. Res., 4, 240 (1971). Reprinted by permission of the American
Chemical Society.
a From equilibrium constant measurements.
stabilize n complexes. Thus 1,2,3,5-tetramethylbenzene forms a complexes that
are 2 billion times more stable than those of toluene, but its .rr complexes are only
more stable than those of toluene by a factor of 3. The rate of bromination of
benzene derivatives also increases drastically with methyl substitution; and the
relative rates are very similar to the relative stabilities of the a complexes.
Apparently the transition state resembles the a complex. As Table 7.16 shows,
there is also a close correlation between rates of chlorination in acetic acid and a
complex stability.
Now that we have determined that the intermediate in electrophilic aro-
matic substitution is usually a a complex (see, however, p. 394), let us return to a
consideration of Reaction 7.76. Two factors probably combine to cause the
observed isotope effect and base catalysis. First, the strong electron-donating
groups stabilize the intermediate 76 (Equation 7.77) and make departure of the
proton more difficult than proton loss in many other electrophilic substitutions.
[Remember, however, that k, < k, (see p. 386).] Second, steric interactions
between the large diazonium group and the nearby substituents increase the rate