Page 404 - Mechanism and Theory in Organic Chemistry
P. 404
Electrophilic Aromatic Substitution 391
decrease the rate (except for the halogens, see Problem 7.1 1) direct the electro-
phile predominantly to the meta position. To understand why this is so, we must
consider the nature of the transition state, but since the transition state is often
similar to the a complex we shall use the o complex as a model for the transition
state.
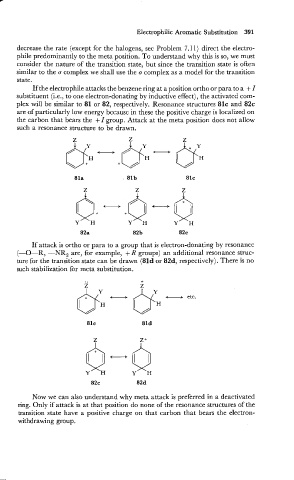
If the electrophile attacks the benzene ring at a position ortho or para to a + I
substituent (i.e., to one electron-donating by inductive effect), the activated com-
plex will be similar to 81 or 82, respectively. Resonance structures 81c and 82c
are of particularly low energy because in these the positive charge is localized on
the carbon that bears the +I group. Attack at the meta position does not allow
such a resonance structure to be drawn.
If attack is ortho or para to a group that is electron-donating by resonance
(-0-R, -NR, are, for example, + R groups) an additional resonance struc-
ture for the transition state can be drawn (81d or 82d, respectively). There is no
such stabilization for meta substitution.
Now we can also understand why meta attack is preferred in a deactivated
ring. Only if attack is at that position do none of the resonance structures of the
transition state have a positive charge on that carbon that bears the electron-
withdrawing group.