Page 96 - Mechanism and Theory in Organic Chemistry
P. 96
Solutions 85
assume that the substance of interest is continuous, with no microscopic struc-
ture. Electrostatic attractions and repulsions between ions are smaller the higher
the dielectric constant, and ions of opposite charge therefore have a greater ten-
dency to dissociate when the dielectric constant is larger. Table 2.11 lists dielec-
tric constants for some common solvents.
The dielectric constant gives only a rough guide to solvent properties, and
does not correlate well with measured effects of solvents on reaction rates. It is
nevertheless useful for making a division of solvents into two broad categories:
polar and nonpolar. In nonpolar solvents, E < - 15, ionic substances will be
highly associated. Indeed, they will be very sparingly soluble in most of these
solvents except as, for example in the case of acetic acid, when hydrogen bonding
is available, and even then solubility is low. Ionic substances are more soluble in
solvents of high dielectric constant, and the ions are dissociated.
Dipole Moment and Polarizability
In order to gain a better understanding of solution phenomena, it is necessary to
evaluate solvent properties on the ,molecular level. Here the most important
properties are the dipole moment, p, and the molecular polarizability. Values
are listed in Table 2.1 1.
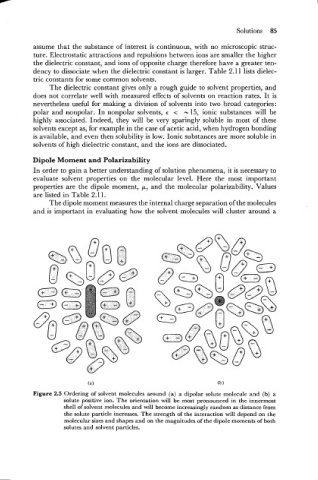
The dipole moment measures the internal charge separation of the molecules
and is important in evaluating how the solvent molecules will cluster around a
Figure 2.5 Ordering of solvent molecules around (a) a dipolar solute molecule and (b) a
solute positive ion. The orientation will be most pronounced in the innermost
shell of solvent molecules and will become increasingly random as distance from
the solute particle increases. The strength of the interaction will depend on the
molecular sizes and shapes and on the magnitudes of the dipole moments of both
solutes and solvent particles.