Page 97 - Mechanism and Theory in Organic Chemistry
P. 97
solute particle that itself has a dipole moment or a net charge. The solvent di-
poles will tend to orient themselves around the solute in the manner indicated
in Figure 2.5. The first solvent layer will be the most highly ordered, with ran-
domness increasing as the influence of the solute particle decreases with increasing
distance. A smaller solute ion will generate a more intense electric field than will
a large one, and so will have a stronger and more far-ranging capacity to orient
solvent dipoles around it. In the solvent molecule itself, one end of the dipole may
be exposed while the other end is buried inside the bulk of the molecule. In
dimethylsulfoxide, for instance, the negative oxygen end of the dipole is exposed
(27), whereas the positive end is not; this solvent interacts much more strongly
with the cations of an ionic solute than with the anions. Formation of solvation
layers around the solute particles will be accompanied by heat evolution (nega-
tive AH) and an increase in order (negative AS).
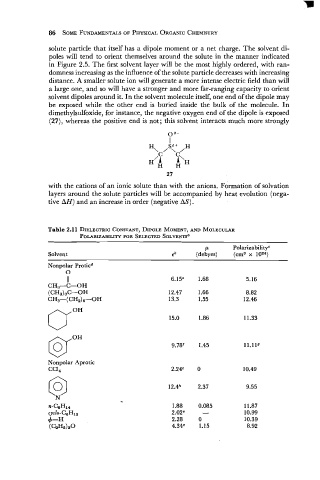
Table 2.11 DIELECTRIC CONSTANT, DIPOLE MOMENT, AND MOLECULAR
POLARIZAB~LITY SELECTED SOLVENTS'
FOR
P PolarizabilityC
Solvent eb (debyes) (cm3 x loz4)
Nonpolar Proticd
0
11 6.15@ 1.68 5.16
CH3-C-OH
(CH3)3C-OH 12.47 1.66 8.82
CH3-(CH2)6-OH 13.3 1.55 12.46
Nonpolar Aprotic
CCl,