Page 98 - Mechanism and Theory in Organic Chemistry
P. 98
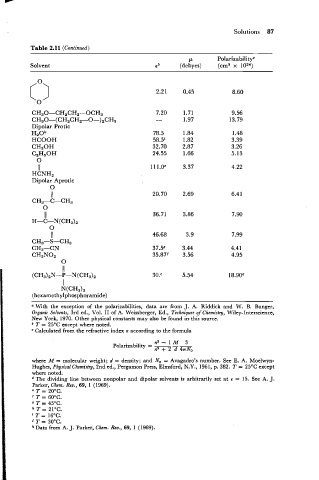
Solutions 87
Table 2.1 1 (Continued)
P Polarizabilityc
Solvent eb (debyes) (cm3 x loz4)
CH30-CH2CH2-OCH3
CH30-(CH2CH2-O-)2CH3
Dipolar Protic
H20k
HCOOH
CH30H
C2H,0H
0
II
HCNH,
Dipolar Aprolic
0
N(CH3)z
(hexamethylphosphoramide)
" With the exception of the polarizabilities, data are from J. A. Riddick and W. B. Bunger,
Organic Solvents, 3rd ed., Vol. I1 of A. Weissberger, Ed., Techniques of Chemistry, Wiley-Interscience,
New York, 1970. Other physical constants may also be found in this source.
T = 25OC except where noted.
Calculated from the refractive index n according to the formula
na-lM 3
Polarizability = - - -
na + 2 d 4vNO
where M = molecular weight; d = density; and No = Avagadro's number. See E. A. Moelwyn-
Hughes, Physical Chemistry, 2nd ed., Pergamon Press, Elmsford, N.Y., 1961, p. 382. T = 25OC except
where noted.
The dividing line between nonpolar and dipolar solvents is arbitrarily set at r = 15. See A. J.
Parker, Chcm. Rev., 69, 1 (1 969).
' T = 20°C.
' T = 60°C.
T = 45°C.
" 21°C.
=
' T = 16OC.
j T = 30°C.
* Data from A. J. Parker, Chcm. Rev., 69, 1 (1969).