Page 33 - Methods For Monitoring And Diagnosing The Efficiency Of Catalytic Converters A Patent - oriented Survey
P. 33
Introduction 15
ratios but they cannot control the emissions of NO, since they must operate at stoichiometric
aidhe1 ratios to obtain simultaneous control of all emissions. A great effort is put on the
development of converters that can control NO, emissions with lean calibrations. The
development of a catalyst system that could control NO, under a lean operation of a diesel
engine ( 14S<air/fiel ratio<22) would be a significant breakthrough (lean NO, catalytic
converter).
The new catalysts are based on zeolites containing active metals exchanged within the zeolite
structure e.g. zeolite converters containing platinum, rhodium and iridium (see e.g. [lo]).
A recent trend to control exhaust emissions is to install a NOx absorbent downstream of the
three-way catalytic converter. This absorbs NO, when the engine operates with a lean air/fbel
ratio and releases NO, when the engine operates with a rich aidfuel ratio. The CO and HC
passing through the three-way catalytic converter during the rich operation reduce then the
NO, released from the absorbent (see e.g. EP0627548 (1994), US5388403 (1995)).
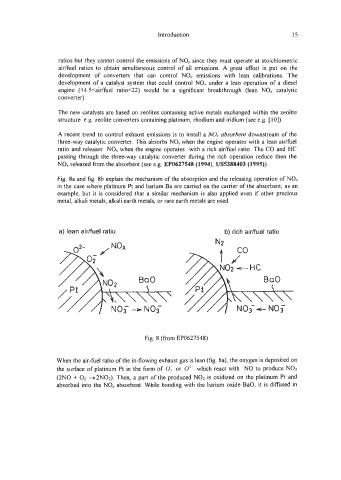
Fig. 8a and fig. 8b explain the mechanism of the absorption and the releasing operation of NO,
in the case where platinum Pt and barium Ba are carried on the carrier of the absorbent, as an
example, but it is considered that a similar mechanism is also applied even if other precious
metal, alkali metals, alkali earth metals, or rare earth metals are used.
a) lean aidfuel ratio b) rich aidfuel ratio
Fig. 8 (fiom EP0627548)
When the air-fuel ratio of the in-flowing exhaust gas is lean (fig. Sa), the oxygen is deposited on
the surface of platinum Pt in the form of 0; or 02- which react with NO to produce NO;!
(2N0 + 02 +2N02). Then, a part of the produced NO2 is oxidized on the platinum Pt and
absorbed into the NO, absorbent. While bonding with the barium oxide BaO, it is diffised in