Page 196 - Modeling of Chemical Kinetics and Reactor Design
P. 196
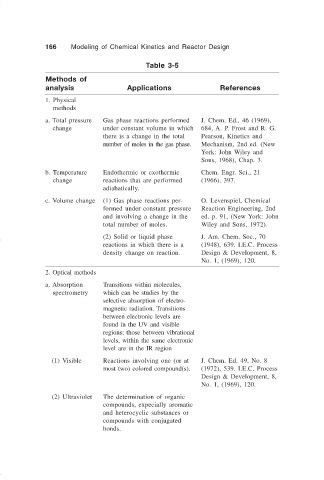
166 Modeling of Chemical Kinetics and Reactor Design
Table 3-5
Methods of
analysis Applications References
1. Physical
methods
a. Total pressure Gas phase reactions performed J. Chem. Ed., 46 (1969),
a. change under constant volume in which 684, A. P. Frost and R. G.
there is a change in the total Pearson, Kinetics and
number of moles in the gas phase. Mechanism, 2nd ed. (New
York: John Wiley and
Sons, 1968), Chap. 3.
b. Temperature Endothermic or exothermic Chem. Engr. Sci., 21
b. change reactions that are performed (1966), 397.
adiabatically.
c. Volume change (1) Gas phase reactions per- O. Levenspiel, Chemical
formed under constant pressure Reaction Engineering, 2nd
and involving a change in the ed. p. 91, (New York: John
total number of moles. Wiley and Sons, 1972).
(2) Solid or liquid phase J. Am. Chem. Soc., 70
reactions in which there is a (1948), 639. I.E.C. Process
density change on reaction. Design & Development, 8,
No. 1, (1969), 120.
2. Optical methods
a. Absorption Transitions within molecules,
a. spectrometry which can be studies by the
selective absorption of electro-
magnetic radiation. Transitions
between electronic levels are
found in the UV and visible
regions; those between vibrational
levels, within the same electronic
level are in the IR region
(1) Visible Reactions involving one (or at J. Chem. Ed. 49, No. 8
most two) colored compound(s). (1972), 539. I.E.C. Process
Design & Development, 8,
No. 1, (1969), 120.
(2) Ultraviolet The determination of organic
compounds, expecially aromatic
and heterocyclic substances or
compounds with conjugated
bonds.