Page 34 - Modeling of Chemical Kinetics and Reactor Design
P. 34
4 Modeling of Chemical Kinetics and Reactor Design
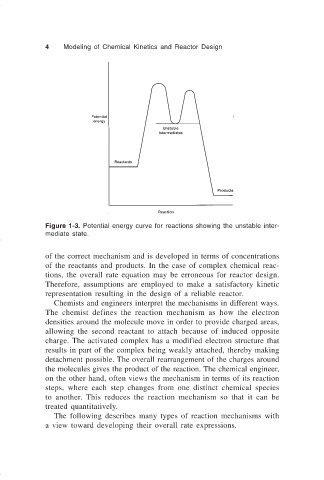
Figure 1-3. Potential energy curve for reactions showing the unstable inter-
mediate state.
of the correct mechanism and is developed in terms of concentrations
of the reactants and products. In the case of complex chemical reac-
tions, the overall rate equation may be erroneous for reactor design.
Therefore, assumptions are employed to make a satisfactory kinetic
representation resulting in the design of a reliable reactor.
Chemists and engineers interpret the mechanisms in different ways.
The chemist defines the reaction mechanism as how the electron
densities around the molecule move in order to provide charged areas,
allowing the second reactant to attach because of induced opposite
charge. The activated complex has a modified electron structure that
results in part of the complex being weakly attached, thereby making
detachment possible. The overall rearrangement of the charges around
the molecules gives the product of the reaction. The chemical engineer,
on the other hand, often views the mechanism in terms of its reaction
steps, where each step changes from one distinct chemical species
to another. This reduces the reaction mechanism so that it can be
treated quantitatively.
The following describes many types of reaction mechanisms with
a view toward developing their overall rate expressions.