Page 32 - Modeling of Chemical Kinetics and Reactor Design
P. 32
2 Modeling of Chemical Kinetics and Reactor Design
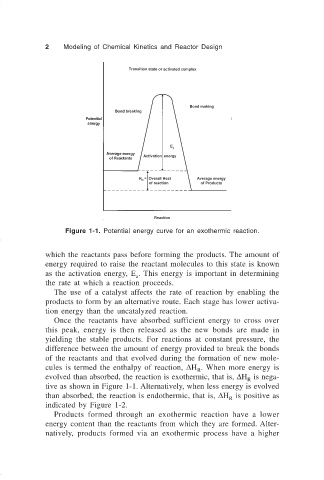
Figure 1-1. Potential energy curve for an exothermic reaction.
which the reactants pass before forming the products. The amount of
energy required to raise the reactant molecules to this state is known
as the activation energy, E . This energy is important in determining
a
the rate at which a reaction proceeds.
The use of a catalyst affects the rate of reaction by enabling the
products to form by an alternative route. Each stage has lower activa-
tion energy than the uncatalyzed reaction.
Once the reactants have absorbed sufficient energy to cross over
this peak, energy is then released as the new bonds are made in
yielding the stable products. For reactions at constant pressure, the
difference between the amount of energy provided to break the bonds
of the reactants and that evolved during the formation of new mole-
cules is termed the enthalpy of reaction, ∆H . When more energy is
R
evolved than absorbed, the reaction is exothermic, that is, ∆H is nega-
R
tive as shown in Figure 1-1. Alternatively, when less energy is evolved
than absorbed, the reaction is endothermic, that is, ∆H is positive as
R
indicated by Figure 1-2.
Products formed through an exothermic reaction have a lower
energy content than the reactants from which they are formed. Alter-
natively, products formed via an exothermic process have a higher