Page 399 - Modelling in Transport Phenomena A Conceptual Approach
P. 399
9.4. MASS TRANSFER WITHOUT CONVECTION 379
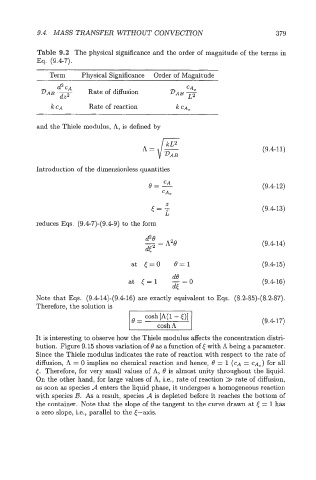
Table 9.2 The physical significance and the order of magnitude of the terms in
Eq. (9.47).
Term Physical Significance Order of Magnitude
CAO
&
-&T Rate of diffusion DAB ~2
CA Rate of reaction k CA,
and the Thiele modulus, A, is defined by
A = J""' (9.411)
DAB
Introduction of the dimensionless quantities
e=- (9.412)
CA
CAO
z
E=, (9.413)
reduces Eqs. (9.47)-(9.49) to the form
&e (9.414)
-- A20
e2 -
at E=O 0=1 (9 A-15)
(9.416)
Note that Eqs. (9.414)-(9.416) are exactly equivalent to Eqs. (8.285)-(8.287).
Therefore, the solution is
(9.417)
It is interesting to observe how the Thiele modulus affects the concentration distri-
bution. Figure 9.15 shows variation of 8 as a function of E with A being a parameter.
Since the Thiele modulus indicates the rate of reaction with respect to the rate of
diffusion, A = 0 implies no chemical reaction and hence, 9 = 1 (CA = CA,) for all
5. Therefore, for very small values of A, 0 is almost unity throughout the liquid.
On the other hand, for large values of A, i.e., rate of reaction >> rate of diffusion,
as soon as species A enters the liquid phase, it undergoes a homogeneous reaction
with species B. As a result, species A is depleted before it reaches the bottom of
the container. Note that the slope of the tangent to the curve drawn at 5 = 1 has
a zero slope, i.e., parallel to the 5-axis.