Page 401 - Modelling in Transport Phenomena A Conceptual Approach
P. 401
9.4. MASS TRANSFER WITHOUT CONVECTION 381
9.4.2 Diffusion in a Spherical Particle With Homogeneous
Reaction
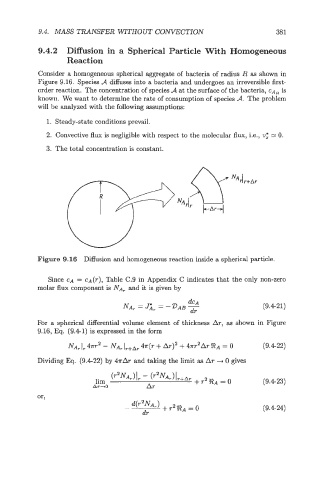
Consider a homogeneous spherical aggregate of bacteria of radius R as shown in
Figure 9.16. Species A diffuses into a bacteria and undergoes an irreversible first-
order reaction. The concentration of species A at the surface of the bacteria, CA~ is
known. We want to determine the rate of consumption of species A. The problem
will be analyzed with the following assumptions:
1. Steady-state conditions prevail.
2. Convective flux is negligible with respect to the molecular flux, i.e., v,* II 0.
3. The total concentration is constant.
Figure 9.16 Diffusion and homogeneous reaction inside a spherical particle.
Since CA = CA(?‘), Table C.9 in Appendix C indicates that the only non-zero
molar flux component is NA, and it is given by
NA, = Jir = --DAB - (9.421)
dCA
dr
For a spherical differential volume element of thickness AT, as shown in Figure
9.16, Eq. (9.41) is expressed in the form
1,.
NA, 47w2 - NA,I,.+A, 4747- + AT)^ + 47rr2Ar %A = 0 (9.422)
Dividing Eq. (9.422) by 47rAr and taking the limit as Ar + 0 gives
or,
- d(r2NAr) + ,.2 %A = (9.424)
dr