Page 253 - Modern Analytical Chemistry
P. 253
1400-CH08 9/9/99 2:17 PM Page 236
236 Modern Analytical Chemistry
0.00
–1.00
–2.00
log (Solubility) –3.00
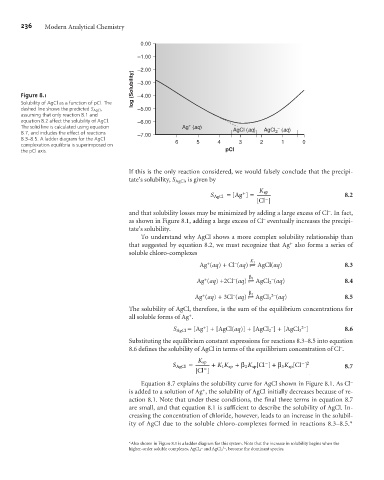
Figure 8.1 –4.00
Solubility of AgCl as a function of pCl. The
dashed line shows the predicted S AgCl , –5.00
assuming that only reaction 8.1 and
equation 8.2 affect the solubility of AgCl. –6.00
+
The solid line is calculated using equation Ag (aq) AgCl (aq) AgCl (aq)
–
8.7, and includes the effect of reactions –7.00 2
8.3–8.5. A ladder diagram for the AgCl 6 5 4 3 2 1 0
complexation equilibria is superimposed on
the pCl axis. pCl
If this is the only reaction considered, we would falsely conclude that the precipi-
tate’s solubility, S AgCl , is given by
K sp
+
S AgCl =[ Ag ] = 8.2
-
[ Cl ]
–
and that solubility losses may be minimized by adding a large excess of Cl . In fact,
–
as shown in Figure 8.1, adding a large excess of Cl eventually increases the precipi-
tate’s solubility.
To understand why AgCl shows a more complex solubility relationship than
+
that suggested by equation 8.2, we must recognize that Ag also forms a series of
soluble chloro-complexes
K 1
–
+
Ag (aq)+Cl (aq) t AgCl(aq) 8.3
b 2
+
–
–
Ag (aq) +2Cl (aq) t AgCl 2 (aq) 8.4
b 3
–
+
2–
Ag (aq) + 3Cl (aq) t AgCl 3 (aq) 8.5
The solubility of AgCl, therefore, is the sum of the equilibrium concentrations for
+
all soluble forms of Ag .
–
2–
+
S AgCl = [Ag ] + [AgCl(aq)] + [AgCl 2 ] + [AgCl 3 ] 8.6
Substituting the equilibrium constant expressions for reactions 8.3–8.5 into equation
–
8.6 defines the solubility of AgCl in terms of the equilibrium concentration of Cl .
K sp - -2
S AgCl = - + 1 K sp Cl[ ] + 3 b K sp Cl[ ] 8.7
KK sp + 2 b
[ Cl ]
Equation 8.7 explains the solubility curve for AgCl shown in Figure 8.1. As Cl –
+
is added to a solution of Ag , the solubility of AgCl initially decreases because of re-
action 8.1. Note that under these conditions, the final three terms in equation 8.7
are small, and that equation 8.1 is sufficient to describe the solubility of AgCl. In-
creasing the concentration of chloride, however, leads to an increase in the solubil-
ity of AgCl due to the soluble chloro-complexes formed in reactions 8.3–8.5.*
*Also shown in Figure 8.1 is a ladder diagram for this system. Note that the increase in solubility begins when the
–
2–
higher-order soluble complexes, AgCl 2 and AgCl 3 , become the dominant species.