Page 254 - Modern Analytical Chemistry
P. 254
1400-CH08 9/9/99 2:17 PM Page 237
Chapter 8 Gravimetric Methods of Analysis 237
pH
14
PO 4 3–
pK = 12.35 H PO
2– 12 a3 3 4
HPO 4
10
HPO 4 2– 8 log(solubility) Ca 3 (PO 4 ) 2 H PO 4 –
2
pK a2 = 7.20
H PO 4 – 6
2
HPO 4 2–
PO 4 3–
4
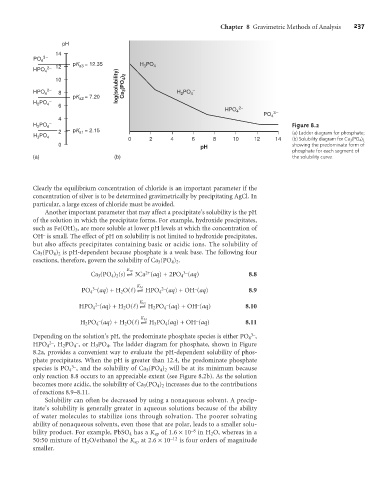
H PO 4 – Figure 8.2
2
2 pK a1 = 2.15 (a) Ladder diagram for phosphate;
H PO 4
3
0 2 4 6 8 10 12 14 (b) Solubility diagram for Ca 3 (PO 4 ) 2
0 pH showing the predominate form of
phosphate for each segment of
(a) (b) the solubility curve.
Clearly the equilibrium concentration of chloride is an important parameter if the
concentration of silver is to be determined gravimetrically by precipitating AgCl. In
particular, a large excess of chloride must be avoided.
Another important parameter that may affect a precipitate’s solubility is the pH
of the solution in which the precipitate forms. For example, hydroxide precipitates,
such as Fe(OH) 3 , are more soluble at lower pH levels at which the concentration of
–
OH is small. The effect of pH on solubility is not limited to hydroxide precipitates,
but also affects precipitates containing basic or acidic ions. The solubility of
Ca 3 (PO 4 ) 2 is pH-dependent because phosphate is a weak base. The following four
reactions, therefore, govern the solubility of Ca 3 (PO 4 ) 2 .
K sp
2+
3–
Ca 3 (PO 4 ) 2 (s) t 3Ca (aq) + 2PO 4 (aq) 8.8
K b1
–
2–
3–
PO 4 (aq)+H 2 O(l) t HPO 4 (aq)+OH (aq) 8.9
K b2
–
–
2–
HPO 4 (aq)+H 2 O(l) t H 2 PO 4 (aq)+OH (aq) 8.10
K b3
–
–
H 2 PO 4 (aq)+H 2 O(l) t H 3 PO 4 (aq)+OH (aq) 8.11
3–
Depending on the solution’s pH, the predominate phosphate species is either PO 4 ,
2–
–
HPO 4 , H 2 PO 4 , or H 3 PO 4 . The ladder diagram for phosphate, shown in Figure
8.2a, provides a convenient way to evaluate the pH-dependent solubility of phos-
phate precipitates. When the pH is greater than 12.4, the predominate phosphate
3–
species is PO 4 , and the solubility of Ca 3 (PO 4 ) 2 will be at its minimum because
only reaction 8.8 occurs to an appreciable extent (see Figure 8.2b). As the solution
becomes more acidic, the solubility of Ca 3 (PO 4 ) 2 increases due to the contributions
of reactions 8.9–8.11.
Solubility can often be decreased by using a nonaqueous solvent. A precip-
itate’s solubility is generally greater in aqueous solutions because of the ability
of water molecules to stabilize ions through solvation. The poorer solvating
ability of nonaqueous solvents, even those that are polar, leads to a smaller solu-
bility product. For example, PbSO 4 has a K sp of 1.6 ´10 –8 in H 2 O, whereas in a
50:50 mixture of H 2 O/ethanol the K sp at 2.6 ´10 –12 is four orders of magnitude
smaller.