Page 259 - Modern Analytical Chemistry
P. 259
1400-CH08 9/9/99 2:17 PM Page 242
242 Modern Analytical Chemistry
NO 3 – OH – Primary
NO 3 – adsorption
NO 3 – layer
Ag +
Ag + NO 3 –
AgCl Ag +
ClAgCl + –
Ag Cl NO –
AgClAgCl Ag + OH – 3 Bulk solution
ClAgClAgCl
Precipitate AgClAgClAgCl Ag + NO 3 –
ClAgClAgClAg
AgClAgClAgCl
Ag +
ClAgClAgCl
Ag + NO –
AgClAgClAg 3
ClAgClAgCl
Ag + NO –
AgClAgCl 3 –
Ag + OH
Ag + NO –
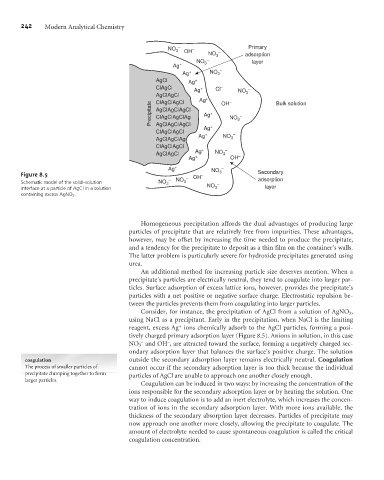
Figure 8.5 OH – 3 Secondary
Schematic model of the solid–solution NO 3 – NO 3 – – adsorption
interface at a particle of AgCl in a solution NO 3 layer
containing excess AgNO 3 .
Homogeneous precipitation affords the dual advantages of producing large
particles of precipitate that are relatively free from impurities. These advantages,
however, may be offset by increasing the time needed to produce the precipitate,
and a tendency for the precipitate to deposit as a thin film on the container’s walls.
The latter problem is particularly severe for hydroxide precipitates generated using
urea.
An additional method for increasing particle size deserves mention. When a
precipitate’s particles are electrically neutral, they tend to coagulate into larger par-
ticles. Surface adsorption of excess lattice ions, however, provides the precipitate’s
particles with a net positive or negative surface charge. Electrostatic repulsion be-
tween the particles prevents them from coagulating into larger particles.
Consider, for instance, the precipitation of AgCl from a solution of AgNO 3 ,
using NaCl as a precipitant. Early in the precipitation, when NaCl is the limiting
+
reagent, excess Ag ions chemically adsorb to the AgCl particles, forming a posi-
tively charged primary adsorption layer (Figure 8.5). Anions in solution, in this case
–
–
NO 3 and OH , are attracted toward the surface, forming a negatively charged sec-
ondary adsorption layer that balances the surface’s positive charge. The solution
coagulation outside the secondary adsorption layer remains electrically neutral. Coagulation
The process of smaller particles of cannot occur if the secondary adsorption layer is too thick because the individual
precipitate clumping together to form particles of AgCl are unable to approach one another closely enough.
larger particles.
Coagulation can be induced in two ways: by increasing the concentration of the
ions responsible for the secondary adsorption layer or by heating the solution. One
way to induce coagulation is to add an inert electrolyte, which increases the concen-
tration of ions in the secondary adsorption layer. With more ions available, the
thickness of the secondary absorption layer decreases. Particles of precipitate may
now approach one another more closely, allowing the precipitate to coagulate. The
amount of electrolyte needed to cause spontaneous coagulation is called the critical
coagulation concentration.