Page 260 - Modern Analytical Chemistry
P. 260
1400-CH08 9/9/99 2:17 PM Page 243
Chapter 8 Gravimetric Methods of Analysis 243
(b) (c)
(a)
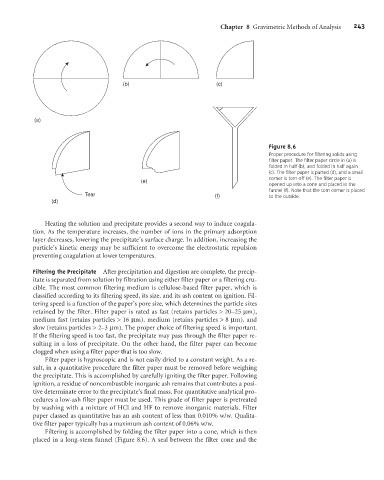
Figure 8.6
Proper procedure for filtering solids using
filter paper. The filter paper circle in (a) is
folded in half (b), and folded in half again
(c). The filter paper is parted (d), and a small
corner is torn off (e). The filter paper is
(e)
opened up into a cone and placed in the
funnel (f). Note that the torn corner is placed
Tear (f) to the outside.
(d)
Heating the solution and precipitate provides a second way to induce coagula-
tion. As the temperature increases, the number of ions in the primary adsorption
layer decreases, lowering the precipitate’s surface charge. In addition, increasing the
particle’s kinetic energy may be sufficient to overcome the electrostatic repulsion
preventing coagulation at lower temperatures.
Filtering the Precipitate After precipitation and digestion are complete, the precip-
itate is separated from solution by filtration using either filter paper or a filtering cru-
cible. The most common filtering medium is cellulose-based filter paper, which is
classified according to its filtering speed, its size, and its ash content on ignition. Fil-
tering speed is a function of the paper’s pore size, which determines the particle sizes
retained by the filter. Filter paper is rated as fast (retains particles > 20–25 mm),
medium fast (retains particles > 16 mm), medium (retains particles > 8 mm), and
slow (retains particles > 2–3 mm). The proper choice of filtering speed is important.
If the filtering speed is too fast, the precipitate may pass through the filter paper re-
sulting in a loss of precipitate. On the other hand, the filter paper can become
clogged when using a filter paper that is too slow.
Filter paper is hygroscopic and is not easily dried to a constant weight. As a re-
sult, in a quantitative procedure the filter paper must be removed before weighing
the precipitate. This is accomplished by carefully igniting the filter paper. Following
ignition, a residue of noncombustible inorganic ash remains that contributes a posi-
tive determinate error to the precipitate’s final mass. For quantitative analytical pro-
cedures a low-ash filter paper must be used. This grade of filter paper is pretreated
by washing with a mixture of HCl and HF to remove inorganic materials. Filter
paper classed as quantitative has an ash content of less than 0.010% w/w. Qualita-
tive filter paper typically has a maximum ash content of 0.06% w/w.
Filtering is accomplished by folding the filter paper into a cone, which is then
placed in a long-stem funnel (Figure 8.6). A seal between the filter cone and the