Page 265 - Modern Analytical Chemistry
P. 265
1400-CH08 9/9/99 2:18 PM Page 248
248 Modern Analytical Chemistry
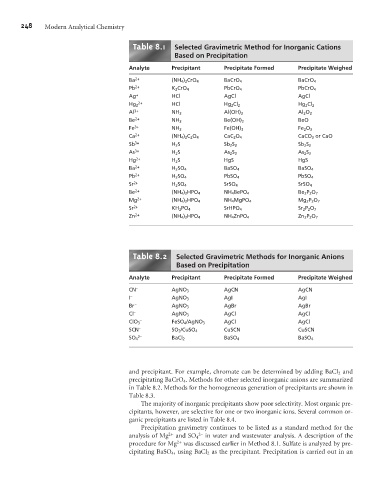
Table 8.1 Selected Gravimetric Method for Inorganic Cations
Based on Precipitation
Analyte Precipitant Precipitate Formed Precipitate Weighed
Ba 2+ (NH 4 ) 2 CrO 4 BaCrO 4 BaCrO 4
Pb 2+ K 2 CrO 4 PbCrO 4 PbCrO 4
Ag + HCl AgCl AgCl
2+ HCl
Hg 2 Hg 2 Cl 2 Hg 2 Cl 2
Al 3+ NH 3 Al(OH) 3 Al 2 O 3
Be 2+ NH 3 Be(OH) 2 BeO
Fe 3+ NH 3 Fe(OH) 3 Fe 2 O 3
Ca 2+ (NH 4 ) 2 C 2 O 4 CaC 2 O 4 CaCO 3 or CaO
Sb 3+ H 2 S Sb 2 S 3 Sb 2 S 3
As 3+ H 2 S As 2 S 3 As 2 S 3
Hg 2+ H 2 S HgS HgS
Ba 2+ H 2 SO 4 BaSO 4 BaSO 4
Pb 2+ H 2 SO 4 PbSO 4 PbSO 4
Sr 2+ H 2 SO 4 SrSO 4 SrSO 4
Be 2+ (NH 4 ) 2 HPO 4 NH 4 BePO 4 Be 2 P 2 O 7
Mg 2+ (NH 4 ) 2 HPO 4 NH 4 MgPO 4 Mg 2 P 2 O 7
Sr 2+ KH 2 PO 4 SrHPO 4 Sr 2 P 2 O 7
Zn 2+ (NH 4 ) 2 HPO 4 NH 4 ZnPO 4 Zn 2 P 2 O 7
Table 8.2 Selected Gravimetric Methods for Inorganic Anions
Based on Precipitation
Analyte Precipitant Precipitate Formed Precipitate Weighed
CN – AgNO 3 AgCN AgCN
I – AgNO 3 AgI AgI
Br – AgNO 3 AgBr AgBr
Cl – AgNO 3 AgCl AgCl
–
ClO 3 FeSO 4/AgNO 3 AgCl AgCl
SCN – SO 2 /CuSO 4 CuSCN CuSCN
2–
SO 4 BaCl 2 BaSO 4 BaSO 4
and precipitant. For example, chromate can be determined by adding BaCl 2 and
precipitating BaCrO 4 . Methods for other selected inorganic anions are summarized
in Table 8.2. Methods for the homogeneous generation of precipitants are shown in
Table 8.3.
The majority of inorganic precipitants show poor selectivity. Most organic pre-
cipitants, however, are selective for one or two inorganic ions. Several common or-
ganic precipitants are listed in Table 8.4.
Precipitation gravimetry continues to be listed as a standard method for the
analysis of Mg 2+ and SO 4 2– in water and wastewater analysis. A description of the
procedure for Mg 2+ was discussed earlier in Method 8.1. Sulfate is analyzed by pre-
cipitating BaSO 4 , using BaCl 2 as the precipitant. Precipitation is carried out in an