Page 266 - Modern Analytical Chemistry
P. 266
1400-CH08 9/9/99 2:18 PM Page 249
Chapter 8 Gravimetric Methods of Analysis 249
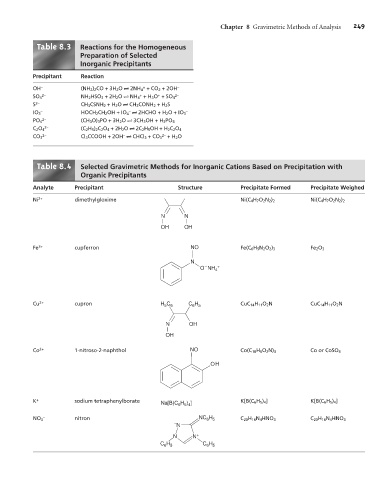
Table 8. 3 Reactions for the Homogeneous
Preparation of Selected
Inorganic Precipitants
Precipitant Reaction
+
OH – (NH 2 ) 2 CO+3H 2 O t 2NH 4 +CO 2 + 2OH –
2– + + 2–
SO 4 NH 2 HSO 3 +2H 2 O t NH 4 +H 3 O +SO 4
S 2– CH 3 CSNH 2 +H 2 O t CH 3 CONH 2 +H 2 S
– – –
IO 3 HOCH 2 CH 2 OH+IO 4 t 2HCHO + H 2 O+IO 3
2–
PO 4 (CH 3 O) 3 PO+3H 2 O t 3CH 3 OH+H 3 PO 4
2–
C 2 O 4 (C 2 H 5 ) 2 C 2 O 4 +2H 2 O t 2C 2 H 5 OH+H 2 C 2 O 4
2– – 2–
CO 3 Cl 3 CCOOH + 2OH t CHCl 3 +CO 3 +H 2 O
Table 8. 4 Selected Gravimetric Methods for Inorganic Cations Based on Precipitation with
Organic Precipitants
Analyte Precipitant Structure Precipitate Formed Precipitate Weighed
Ni 2+ dimethylgloxime Ni(C 4 H 7 O 2 N 2 ) 2 Ni(C 4 H 7 O 2 N 2 ) 2
N N
OH OH
Fe 3+ cupferron NO Fe(C 6 H 5 N 2 O 2 ) 3 Fe 2 O 3
N
–
O NH 4 +
Cu 2+ cupron H C C H CuC 14 H 11 O 2 N CuC 14 H 11 O 2 N
5 6
6 5
N OH
OH
Co 2+ 1-nitroso-2-naphthol NO Co(C 10 H 6 O 2 N) 3 Co or CoSO 4
OH
K + sodium tetraphenylborate Na[B(C H ) ] K[B(C 6 H 5 ) 4 ] K[B(C 6 H 5 ) 4 ]
6 5 4
– NC H
NO 3 nitron 6 5 C 20 H 16 N 4 HNO 3 C 20 H 16 N 4 HNO 3
– N
N N +
H
C 6 5 C H
6 5