Page 267 - Modern Analytical Chemistry
P. 267
1400-CH08 9/9/99 2:18 PM Page 250
250 Modern Analytical Chemistry
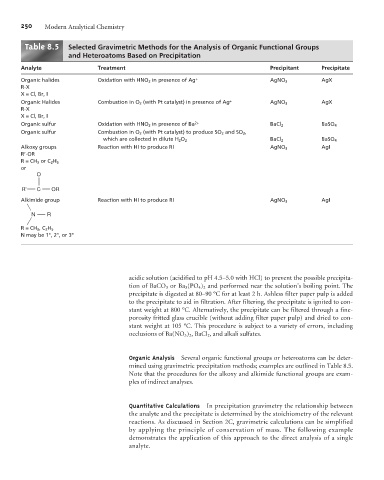
Table 8. 5 Selected Gravimetric Methods for the Analysis of Organic Functional Groups
and Heteroatoms Based on Precipitation
Analyte Treatment Precipitant Precipitate
Organic halides Oxidation with HNO 3 in presence of Ag + AgNO 3 AgX
R-X
X = Cl, Br, I
Organic Halides Combustion in O 2 (with Pt catalyst) in presence of Ag + AgNO 3 AgX
R-X
X = Cl, Br, I
Organic sulfur Oxidation with HNO 3 in presence of Ba 2+ BaCl 2 BaSO 4
Organic sulfur Combustion in O 2 (with Pt catalyst) to produce SO 2 and SO 3 ,
which are collected in dilute H 2 O 2 BaCl 2 BaSO 4
Alkoxy groups Reaction with HI to produce RI AgNO 3 AgI
R’-OR
R=CH 3 or C 2 H 5
or
O
R' C OR
Alkimide group Reaction with HI to produce RI AgNO 3 AgI
N R
R=CH 3 , C 2 H 5
N may be 1°, 2°, or 3°
acidic solution (acidified to pH 4.5–5.0 with HCl) to prevent the possible precipita-
tion of BaCO 3 or Ba 3 (PO 4 ) 2 and performed near the solution’s boiling point. The
precipitate is digested at 80–90 °C for at least 2 h. Ashless filter paper pulp is added
to the precipitate to aid in filtration. After filtering, the precipitate is ignited to con-
stant weight at 800 °C. Alternatively, the precipitate can be filtered through a fine-
porosity fritted glass crucible (without adding filter paper pulp) and dried to con-
stant weight at 105 °C. This procedure is subject to a variety of errors, including
occlusions of Ba(NO 3 ) 2 , BaCl 2 , and alkali sulfates.
Organic Analysis Several organic functional groups or heteroatoms can be deter-
mined using gravimetric precipitation methods; examples are outlined in Table 8.5.
Note that the procedures for the alkoxy and alkimide functional groups are exam-
ples of indirect analyses.
Quantitative Calculations In precipitation gravimetry the relationship between
the analyte and the precipitate is determined by the stoichiometry of the relevant
reactions. As discussed in Section 2C, gravimetric calculations can be simplified
by applying the principle of conservation of mass. The following example
demonstrates the application of this approach to the direct analysis of a single
analyte.