Page 102 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 102
Direct Methanol Fuel Cells
85
-3
with ρ the density of the platinum (21.4 g cm ). The estimated value of
. d can be then compared with the size observed by microscopic techniques.
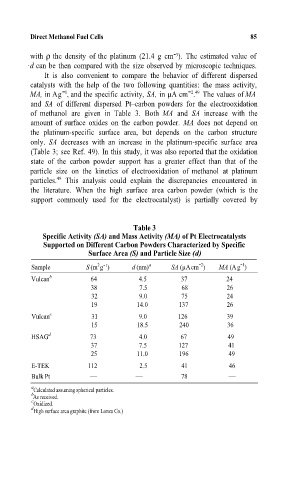
It is also convenient to compare the behavior of different dispersed
catalysts with the help of the two following quantities: the mass activity,
-1
MA, in Ag , and the specific activity, SA, in µA cm . The values of MA
-2 49
and SA of different dispersed Ptcarbon powders for the electrooxidation
of methanol are given in Table 3. Both MA and SA increase with the
amount of surface oxides on the carbon powder. MA does not depend on
the platinum-specific surface area, but depends on the carbon structure
only. SA decreases with an increase in the platinum-specific surface area
(Table 3; see Ref. 49). In this study, it was also reported that the oxidation
state of the carbon powder support has a greater effect than that of the
particle size on the kinetics of electrooxidation of methanol at platinum
particles. 49 This analysis could explain the discrepancies encountered in
the literature. When the high surface area carbon powder (which is the
support commonly used for the electrocatalyst) is partially covered by
Tùle 3A
Specific ActivityA(SA) and Mass ActivityA(MA) of Pt ElectrocatalystsA
Supported on Different Caáon Powders Characterized by SpecificA
Surface AreaA(S) and Particle SizeA(d)
-1
-2
2 -1
Sample S(m g ) d (nm) a SA (µAcm ) MA (Ag )
Vulcan b 64 4.5 37 24
38 7.5 68 26
32 9.0 75 24
19 14.0 137 26
Vulcan c 31 9.0 126 39
15 18.5 240 36
HSAG d 73 4.0 67 49
37 7.5 127 41
25 11.0 196 49
E-TEK 112 2.5 41 46
Bulk Pt 78
a Calculated assuming spherical particles.
b As received.
c Oxidized.
d
High surface area graphite (from Lonza Co.)