Page 103 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 103
Claude LamyAet al.
86
oxidized groups, the strength of the PtO bonds on the particle surface can
be modified. From this point of view, adsorbed OH species are necessary
for the rate-determining step of methanol oxidation; the particle size
effects, as observed in the results of this study, are quite valuable.
Another convenient way to disperse platinum-based electrocatalysts
is to use electron-conducting polymers, such as polyaniline (PAni) or
polypyrrole (PPy), which play the role of a three-dimensional elec-
trode. 50,51 In such a way very dispersed electrocatalysts are obtained, with
particle sizes on the order of a few nanometers, leading to a very high
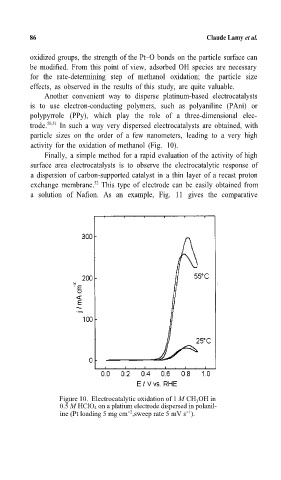
activity for the oxidation of methanol (Fig. 10).
Finally, a simple method for a rapid evaluation of the activity of high
surface area electrocatalysts is to observe the electrocatalytic response of
a dispersion of carbon-supported catalyst in a thin layer of a recast proton
52
exchange membrane. This type of electrode can be easily obtained from
a solution of Nafion. As an example, Fig. 11 gives the comparative
Figure 10. Electrocatalytic oxidation of 1 M CH OH in
3
0.5 M HClO 4 on a platium electrode dispersed in polanil-
-1
ine (Pt loading 5 mg cm ,sweep rate 5 mV s ).
-2