Page 47 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 47
Zbigniew KoczorowskiA
34
phylic lithium chloride in water constitute, within certain potential limits,
polarizablp 1iquid/liquid interfaces. l5–19
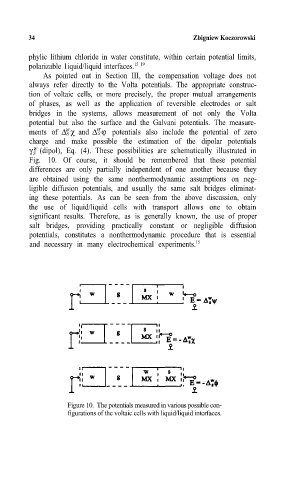
As pointed out in Section III, the compensation voltage does not
always refer directly to the Volta potentials. The appropriate construc-
tion of voltaic cells, or more precisely, the proper mutual arrangements
of phases, as well as the application of reversible electrodes or salt
bridges in the systems, allows measurement of not only the Volta
potential but also the surface and the Galvani potentials.= The measure -
W
ments of ∆ S χ and ∆S ϕ potentials also include the potential of zero
W
charge and m¸p possiblp the estimation of the dipolar potentials
γ (dipol), Eq. (4). These possibilities are schematically illustrated in
W
S
Fig. 10. Of course, it should be remembered that these potential
differences are only partially independent of one another because they
are obtained using the same nonthermodynamic assumptions on neg-
ligible diffusion potentials, and usually the same salt bridges eliminat-
ing these potentials.= As can be seen from the above discussion, only
the use of liquid/liquid cells with transport allows one to obtain
significant results.= Therefore, as is generally known, the use of proper
salt bridges, providing practically constant or negligible diffusion
potentials, constitutes a nonthermodynamic procedure that is essential
and necessaryin many electrochemical experiments. 15
Figure 10. The potentials measured in various possible con-
figurations of the voltaic cells with liquid/liquid interfaces.=