Page 82 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 82
Claude LamyAet al.
66
2
2
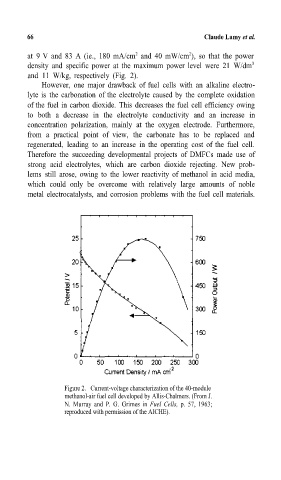
at 9 V and 83 A (ie.,= 180 mA/cm and 40 mW/cm ), so that the power
densityand specific power at the maximum power level were 21 W/dm 3
and 11 W/kg, respectively(Fig. 2).
However, one major drawback of fuel cells with an alkaline electro-
lyte is the carbonation of the electrolyte caused by the complete oxidation
of the fuel in carbon dioxide. This decreases the fuel cell efficiencyowing
to both a decrease in the electrolyte conductivity and an increase in
concentration polarization, mainlyat the oxygen electrode. Furthermore,
from a practical point of view, the carbonate has to be replaced and
regenerated, leading to an increase in the operating cost of the fuel cell.
Therefore the succeeding developmental projects of DMFCs made use of
strong acid electrolytes, which are carbon dioxide rejecting. New prob-
lems still arose, owing to the lower reactivityof methanol in acid media,
which could only be overcome with relatively large amounts of noble
metal electrocatalysts, and corrosion problems with the fuel cell materials.
Figure 2. Current-voltage characterization of the 40-module
methanol-air fuel cell developed by Allis-Chalmers.=(From J.
N.= Murray and P. G.= Grimes in Fuel Cells, p.= 57, 1963;
reproduced with permission of the AICHE).