Page 88 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 88
Direct Methanol Fuel Cells
-3
and W e =W s x ρ = 4.82 kWhr dm , where M = 0.032 kg is the molar 71
-3
weight of methanol and ρ = 0.7914 kg dm is its density.
o
Under the standard reversible conditions (25 C), the energy effi-
ciency is very high:
o ∆G o 702
nFE r
ε rev = ( ∆H ) = ( ∆H ) = ∆H o = 726 =96.7% (8)
W e
_
_
o
o
This is considerably higher than that of an H -O 2 fuel cell (i.e., 83%).
2
However, under normal operating conditions, at a current density j, the
electrode potentials deviate from their equilibrium values as a result of
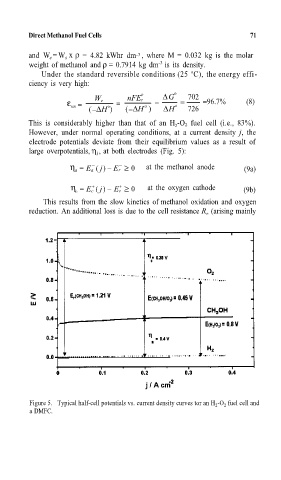
large overpotentials, η , at both electrodes (Fig. 5):
i
η = E a ( j) Er ≥ 0 at the methanol anode (9a)
a
+
η = E c ( j) E r ≥ 0 at the oxygen cathode (9b)
+
c
This results from the slow kinetics of methanol oxidation and oxygen
reduction. An additional loss is due to the cell resistance R e (arising mainly
Figure 5. Typical half-cell potentials vs. current density curves tor an H 2 -O 2 fuel cell and
a DMFC.