Page 94 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 94
Direct Methanol Fuel Cells
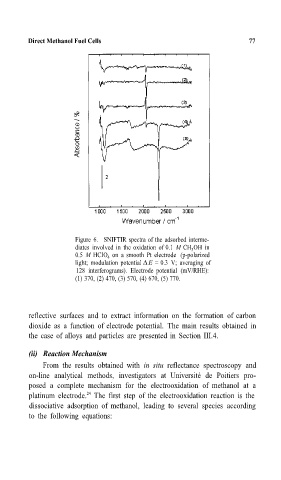
Figure 6. SNIFTIR spectra of the adsorbed interme- 77
diates involved in the oxidation of 0.1 M CH OH in
3
0.5 M HCIO 4 on a smooth Pt electrode (p-polarized
light; modulation potential ∆E = 0.3 V; averaging of
128 interferograms). Electrode potential (mV/RHE):
(1) 370, (2) 470, (3) 570, (4) 670, (5) 770.
reflective surfaces and to extract information on the formation of carbon
dioxide as a function of electrode potential. The main results obtained in
the case of alloys and particles are presented in Section III.4.
(ii) Reaction Mechanism
From the results obtained with in situ reflectance spectroscopy and
on-line analytical methods, investigators at Université de Poitiers pro-
posed a complete mechanism for the electrooxidation of methanol at a
platinum electrode. The first step of the electrooxidation reaction is the
24
dissociative adsorption of methanol, leading to several species according
to the following equations: