Page 95 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 95
Claude LamyAet al.
78
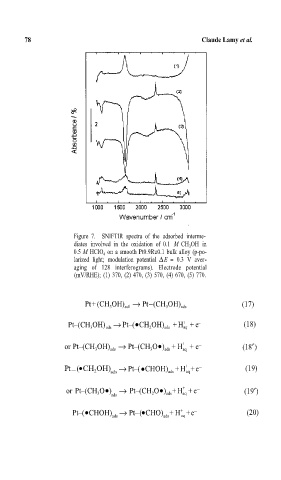
Figure 7. SNIFTIR spectra of the adsorbed interme-
diates involved in the oxidation of 0.1 M CH OH in
3
0.5 M HClO on a smooth Pt0.9Ru0.1 bulk alloy (p-po-
4
larized light; modulation potential ∆E = 0.3 V aver-
aging of 128 interferograms). Electrode potential
(mV/RHE); (1) 370, (2) 470, (3) 570, (4) 670, (5) 770.
_
Pt+ (CH 3 OH) sol → Pt (CH 3 OH) ads (17)
+
Pt(CH OH) → Pt( CH 2 OH) ads +H aq +e (18)
ads
3
+
or Pt(CH 3 OH) ads → Pt(CH O ) +H aq +e (18′)
3 ads
_
+
Pt (. CH OH) ads → Pt( CHOH) +H +e (19)
ads
2
aq
+
or Pt(CH O ) → Pt(CH O ) +H +e (19′)
3 ads 2 ads aq
Pt( CHOH) → Pt( CHO) +H +e (20)
+
aq
ads
ads