Page 177 - MODERN ELECTROCHEMISTRY
P. 177
ION-SOLVENT INTERACTIONS 117
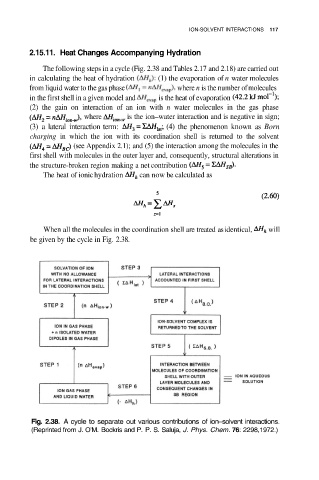
2.15.11. Heat Changes Accompanying Hydration
The following steps in a cycle (Fig. 2.38 and Tables 2.17 and 2.18) are carried out
in calculating the heat of hydration (1) the evaporation of n water molecules
from liquid water to the gas phase where n is the number of molecules
in the first shell in a given model and is the heat of evaporation
(2) the gain on interaction of an ion with n water molecules in the gas phase
where is the ion–water interaction and is negative in sign;
(3) a lateral interaction term: (4) the phenomenon known as Born
charging in which the ion with its coordination shell is returned to the solvent
(see Appendix 2.1); and (5) the interaction among the molecules in the
first shell with molecules in the outer layer and, consequently, structural alterations in
the structure-broken region making a net contribution
The heat of ionic hydration can now be calculated as
When all the molecules in the coordination shell are treated as identical, will
be given by the cycle in Fig. 2.38.
Fig. 2.38. A cycle to separate out various contributions of ion–solvent interactions.
(Reprinted from J. O’M. Bockris and P. P. S. Saluja, J. Phys. Chem. 76: 2298,1972.)