Page 172 - MODERN ELECTROCHEMISTRY
P. 172
112 CHAPTER 2
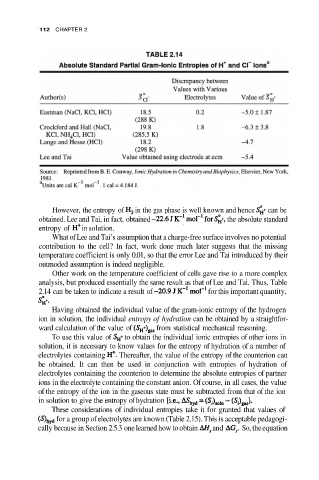
However, the entropy of in the gas phase is well known and hence can be
obtained. Lee and Tai, in fact, obtained the absolute standard
entropy of in solution.
What of Lee and Tai’s assumption that a charge-free surface involves no potential
contribution to the cell? In fact, work done much later suggests that the missing
temperature coefficient is only 0.01, so that the error Lee and Tai introduced by their
outmoded assumption is indeed negligible.
Other work on the temperature coefficient of cells gave rise to a more complex
analysis, but produced essentially the same result as that of Lee and Tai. Thus, Table
2.14 can be taken to indicate a result of for this important quantity,
Having obtained the individual value of the gram-ionic entropy of the hydrogen
ion in solution, the individual entropy of hydration can be obtained by a straightfor-
ward calculation of the value of from statistical mechanical reasoning.
To use this value of to obtain the individual ionic entropies of other ions in
solution, it is necessary to know values for the entropy of hydration of a number of
electrolytes containing Thereafter, the value of the entropy of the counterion can
be obtained. It can then be used in conjunction with entropies of hydration of
electrolytes containing the counterion to determine the absolute entropies of partner
ions in the electrolyte containing the constant anion. Of course, in all cases, the value
of the entropy of the ion in the gaseous state must be subtracted from that of the ion
in solution to give the entropy of hydration
These considerations of individual entropies take it for granted that values of
for a group of electrolytes are known (Table 2.15). This is acceptable pedagogi-
cally because in Section 2.5.3 one learned how to obtain and So, the equation