Page 169 - MODERN ELECTROCHEMISTRY
P. 169
ION–SOLVENT INTERACTIONS 109
the experimental points do give a straight line unless the ionic radius falls below about
13 nm. Further, the theoretical slope is in fair agreement with the
experimental slope
It can therefore be concluded that by considering a quadrupole model for the water
molecule, one can not only explain why oppositely charged ions of equal radius have
differing heats of hydration (Fig. 2.32), but can also quantitatively predict the way
these differences in the heats of hydration will vary with the radius of the ions
concerned.
What are we seeking in this section? The objective is a method to unscramble the
individual heats of hydration from values known for the salt, i.e., for at least two
individual ions.
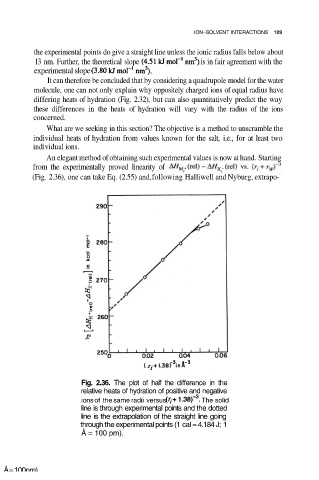
An elegant method of obtaining such experimental values is now at hand. Starting
from the experimentally proved linearity of
(Fig. 2.36), one can take Eq. (2.55) and, following Halliwell and Nyburg, extrapo-
Fig. 2.36. The plot of half the difference in the
relative heats of hydration of positive and negative
ions of the same radii versus The solid
line is through experimental points and the dotted
line is the extrapolation of the straight line going
through the experimental points (1 cal = 4.184 J; 1
Å = 100 pm).
Å=100pm)