Page 164 - MODERN ELECTROCHEMISTRY
P. 164
104 CHAPTER 2
and the total heat of ion–water interactions is
If one scrutinizes the various steps of the cycle, it will be realized that for only
one step, namely, step 3, does the heat content change [Eq. (2.41)] depending upon
whether one views the water molecule as an electrical dipole or quadrupole. Hence,
the expressions for the heat changes for all steps except step 3 can be carried over as
such into the theoretical heat of ion–water interactions, derived earlier. In step
3, onehas to replace the heat of ion–dipole interactions with the heat
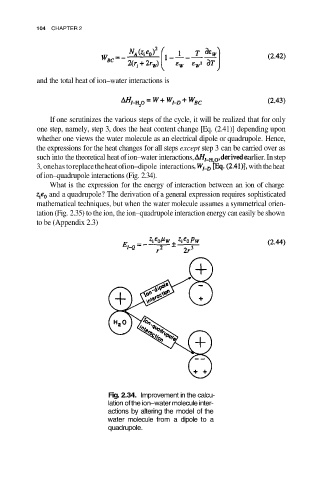
of ion–quadrupole interactions (Fig. 2.34).
What is the expression for the energy of interaction between an ion of charge
and a quadrupole? The derivation of a general expression requires sophisticated
mathematical techniques, but when the water molecule assumes a symmetrical orien-
tation (Fig. 2.35) to the ion, the ion–quadrupole interaction energy can easily be shown
to be (Appendix 2.3)
Fig. 2.34. Improvement in the calcu-
lation of the ion–water molecule inter-
actions by altering the model of the
water molecule from a dipole to a
quadrupole.