Page 167 - MODERN ELECTROCHEMISTRY
P. 167
ION–SOLVENT INTERACTIONS 107
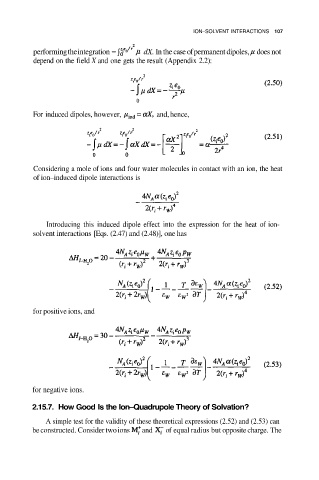
performing the integration dX. In the case of permanent dipoles, does not
depend on the field X and one gets the result (Appendix 2.2):
For induced dipoles, however, and, hence,
Considering a mole of ions and four water molecules in contact with an ion, the heat
of ion–induced dipole interactions is
Introducing this induced dipole effect into the expression for the heat of ion-
solvent interactions [Eqs. (2.47) and (2.48)], one has
for positive ions, and
for negative ions.
2.15.7. How Good Is the lon–Quadrupole Theory of Solvation?
A simple test for the validity of these theoretical expressions (2.52) and (2.53) can
be constructed. Consider two ions and of equal radius but opposite charge. The