Page 165 - MODERN ELECTROCHEMISTRY
P. 165
ION-SOLVENT INTERACTIONS 105
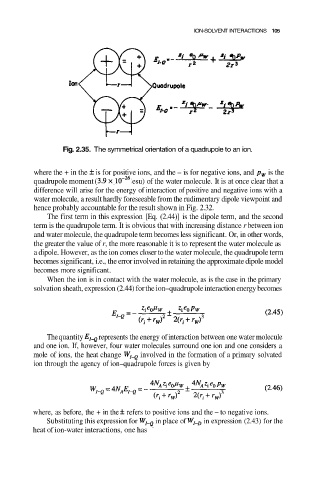
Fig. 2.35. The symmetrical orientation of a quadrupole to an ion.
where the + in the is for positive ions, and the – is for negative ions, and is the
quadrupole moment ( esu) of the water molecule. It is at once clear that a
difference will arise for the energy of interaction of positive and negative ions with a
water molecule, a result hardly foreseeable from the rudimentary dipole viewpoint and
hence probably accountable for the result shown in Fig. 2.32.
The first term in this expression [Eq. (2.44)] is the dipole term, and the second
term is the quadrupole term. It is obvious that with increasing distance r between ion
and water molecule, the quadrupole term becomes less significant. Or, in other words,
the greater the value of r, the more reasonable it is to represent the water molecule as
a dipole. However, as the ion comes closer to the water molecule, the quadrupole term
becomes significant, i.e., the error involved in retaining the approximate dipole model
becomes more significant.
When the ion is in contact with the water molecule, as is the case in the primary
solvation sheath, expression (2.44) for the ion–quadrupole interaction energy becomes
The quantity represents the energy of interaction between one water molecule
and one ion. If, however, four water molecules surround one ion and one considers a
mole of ions, the heat change involved in the formation of a primary solvated
ion through the agency of ion–quadrupole forces is given by
where, as before, the + in the refers to positive ions and the – to negative ions.
Substituting this expression for in place of in expression (2.43) for the
heat of ion-water interactions, one has