Page 162 - MODERN ELECTROCHEMISTRY
P. 162
102 CHAPTER 2
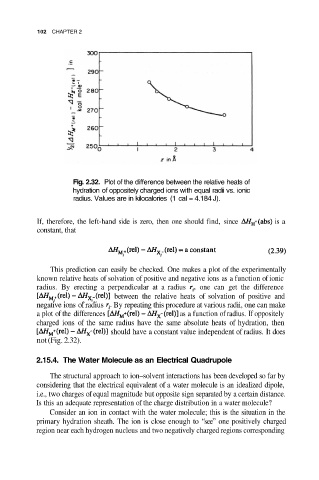
Fig. 2.32. Plot of the difference between the relative heats of
hydration of oppositely charged ions with equal radii vs. ionic
radius. Values are in kilocalories (1 cal = 4.184 J).
If, therefore, the left-hand side is zero, then one should find, since is a
constant, that
This prediction can easily be checked. One makes a plot of the experimentally
known relative heats of solvation of positive and negative ions as a function of ionic
radius. By erecting a perpendicular at a radius one can get the difference
between the relative heats of solvation of positive and
negative ions of radius By repeating this procedure at various radii, one can make
a plot of the differences as a function of radius. If oppositely
charged ions of the same radius have the same absolute heats of hydration, then
should have a constant value independent of radius. It does
not (Fig. 2.32).
2.15.4. The Water Molecule as an Electrical Quadrupole
The structural approach to ion–solvent interactions has been developed so far by
considering that the electrical equivalent of a water molecule is an idealized dipole,
i.e., two charges of equal magnitude but opposite sign separated by a certain distance.
Is this an adequate representation of the charge distribution in a water molecule?
Consider an ion in contact with the water molecule; this is the situation in the
primary hydration sheath. The ion is close enough to “see” one positively charged
region near each hydrogen nucleus and two negatively charged regions corresponding