Page 160 - MODERN ELECTROCHEMISTRY
P. 160
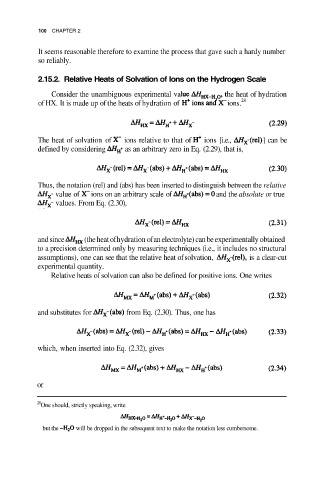
100 CHAPTER 2
It seems reasonable therefore to examine the process that gave such a hardy number
so reliably.
2.15.2. Relative Heats of Solvation of Ions on the Hydrogen Scale
Consider the unambiguous experimental va he heat of hydration
of HX. It is made up of the heats of hydration of ions. 28
The heat of solvation of ions relative to that of ions [i.e., ] can be
defined by considering as an arbitrary zero in Eq. (2.29), that is,
Thus, the notation (rel) and (abs) has been inserted to distinguish between the relative
value of ions on an arbitrary scale of and the absolute or true
values. From Eq. (2.30),
and since (the heat of hydration of an electrolyte) can be experimentally obtained
to a precision determined only by measuring techniques (i.e., it includes no structural
assumptions), one can see that the relative heat of solvation, is a clear-cut
experimental quantity.
Relative heats of solvation can also be defined for positive ions. One writes
and substitutes for from Eq. (2.30). Thus, one has
which, when inserted into Eq. (2.32), gives
or
28
One should, strictly speaking, write
but the will be dropped in the subsequent text to make the notation less cumbersome.