Page 157 - MODERN ELECTROCHEMISTRY
P. 157
ION–SOLVENT INTERACTIONS 97
between 70 and 85% of the heat of hydration of ions comes from the first shell. One
reason for the interest in this result lies in the electrode process field where the
traditional theory of the energetics of electron transfer has in the past stressed the outer
and not the inner shell solvation as having a major influence on electron transfer,
although the present material makes the waters in the first layer those which exert the
major control on the ion–solvent interaction energy.
Kebarle used a pulsed electron beam to produce ions for injection into a mass
spectrograph that contained the water molecules at a determined, but variable partial
pressure. Hiraoka et al. found that decreases more rapidly with a change in the
numbers of water molecules attached to than for and crosses over at
–
Evidence for some degree of covalent bonding occurs for F (CH 3CN). The completion
of the first solvation shell does not occur until in this case, a surprisingly high
number. The trends found are diagrammed in Fig. 2.30.
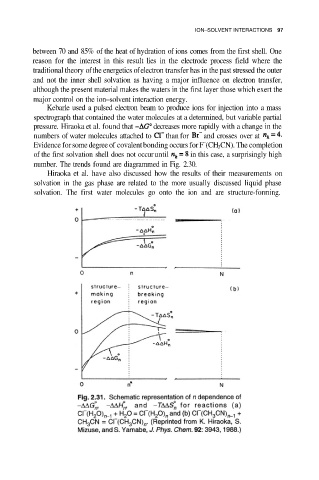
Hiraoka et al. have also discussed how the results of their measurements on
solvation in the gas phase are related to the more usually discussed liquid phase
solvation. The first water molecules go onto the ion and are structure-forming.