Page 152 - MODERN ELECTROCHEMISTRY
P. 152
92 CHAPTER 2
2.12.2. How Does One Measure the Dielectric Constant of Ionic
Solutions?
There is much to think about here. If one wishes to measure the dielectric constant
of a liquid, not a conducting ionic solution, one simply uses an alternating current (ac)
bridge containing a capacitor in one of the arms. Then the capacitance is measured in
the presence of the liquid, the dielectric constant of which is to be measured, and then
without it, i.e., in the presence of air. Since the dielectric constant is near to unity in
the latter case, this gives rise to knowledge of the dielectric constant of the liquid
because the capacitance of the cell in the bridge arm increases as the dielectric constant
increases.
However, when the liquid is a conducting solution, this approach breaks down.
The conductance of the solution contributes to the impedance of the cell, which
(depending on frequency) may no longer be overwhelmingly capacitative. Hence, the
dielectric constant of a conducting liquid cannot be simply measured, because of the
conductive components of the impedance.
Two approaches can be used to avoid this difficulty. In the one, which is best used
for frequencies well below the relaxation frequency of water, one measures the force
between two plates that have the conducting liquid between them. This force is
independent of the conductance of the liquid or the direction of the field. If d is the
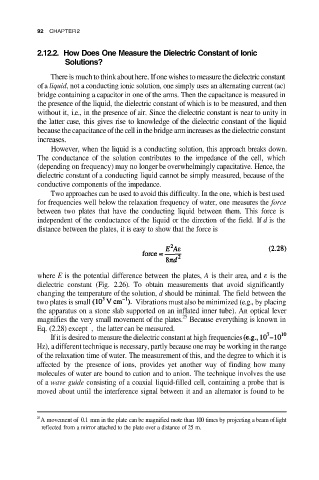
distance between the plates, it is easy to show that the force is
where E is the potential difference between the plates, A is their area, and is the
dielectric constant (Fig. 2.26). To obtain measurements that avoid significantly
changing the temperature of the solution, d should be minimal. The field between the
two plates is small Vibrations must also be minimized (e.g., by placing
the apparatus on a stone slab supported on an inflated inner tube). An optical lever
25
magnifies the very small movement of the plates. Because everything is known in
Eq. (2.28) except , the latter can be measured.
If it is desired to measure the dielectric constant at high frequencies
Hz), a different technique is necessary, partly because one may be working in the range
of the relaxation time of water. The measurement of this, and the degree to which it is
affected by the presence of ions, provides yet another way of finding how many
molecules of water are bound to cation and to anion. The technique involves the use
of a wave guide consisting of a coaxial liquid-filled cell, containing a probe that is
moved about until the interference signal between it and an alternator is found to be
25
A movement of 0.1 mm in the plate can be magnified mote than 100 times by projecting a beam of light
reflected from a mirror attached to the plate over a distance of 25 m.