Page 151 - MODERN ELECTROCHEMISTRY
P. 151
ION–SOLVENT INTERACTIONS 91
The main purpose of this section is to give the basis of how measurements of the
dielectric constants of ionic solutions can give information on solvation, particularly
primary hydration numbers. However, dielectric measurements as a function of
frequency also give information on the dynamic behavior of water by allowing us to
determine the relaxation time of water in ionic solutions and expressing the changes
in terms of the number of water molecules bound to the ion.
Dielectric measurements of ionic solutions are also important for another topic
that will be dealt with in Section 2.22, namely, electrostriction, the study of the
compressive effect of the very strong electric fields produced by ions on the surround-
ing medium. When one looks into the effect of ions on the frequency at which water
undergoes relaxation (i.e., when water no longer reacts to an applied field), it is found
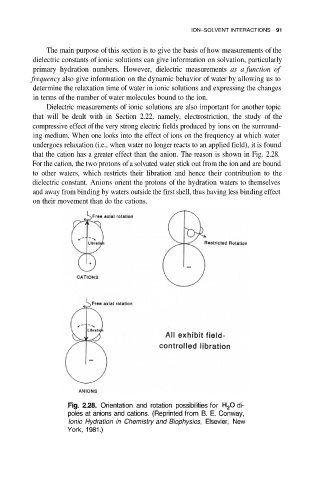
that the cation has a greater effect than the anion. The reason is shown in Fig. 2.28.
For the cation, the two protons of a solvated water stick out from the ion and are bound
to other waters, which restricts their libration and hence their contribution to the
dielectric constant. Anions orient the protons of the hydration waters to themselves
and away from binding by waters outside the first shell, thus having less binding effect
on their movement than do the cations.
Fig. 2.28. Orientation and rotation possibilities for di-
poles at anions and cations. (Reprinted from B. E. Conway,
Ionic Hydration in Chemistry and Biophysics, Elsevier, New
York, 1981.)