Page 149 - MODERN ELECTROCHEMISTRY
P. 149
ION–SOLVENT INTERACTIONS 89
liquid) if ions are added to them. Thus, the ions undergo solvation and, to some critical
distance from the ions’ center, hold the solvent molecules tightly against the tendency
of the field to orient them to oppose the applied field.
Those water molecules that are prevented from orienting (“irrotationally bound”)
to oppose the field will be withdrawn from those contributing to the counter field and
hence the dielectric constant of the ionic solution will be reduced from whatthe solvent
would have without the ions.
There are a number of publications in the field of the structure of ionic solutions
that are particularly seminal and although published half a century ago have great
influence on present concepts. One of them is the paper by Bernal and Fowler in which
the associated structure of water was first established (in 1933) from the interpretation
of the original X-ray data on liquids. However, another paper of great importance is
that by Hasted, Ritson, and Collie in 1948, for it was here that the dielectric properties
of solutions were first recorded on a large scale. In subsequent publications the relation
of the dielectric constant of the solution and solvation was first investigated.
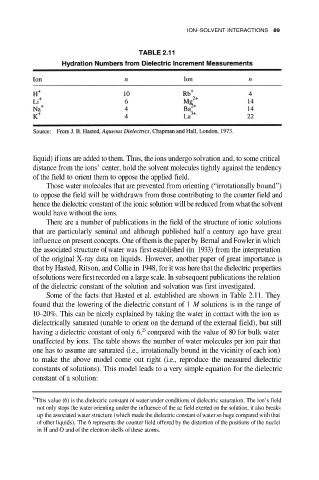
Some of the facts that Hasted et al. established are shown in Table 2.11. They
found that the lowering of the dielectric constant of 1 M solutions is in the range of
10–20%. This can be nicely explained by taking the water in contact with the ion as
dielectrically saturated (unable to orient on the demand of the external field), but still
23
having a dielectric constant of only 6, compared with the value of 80 for bulk water
unaffected by ions. The table shows the number of water molecules per ion pair that
one has to assume are saturated (i.e., irrotationally bound in the vicinity of each ion)
to make the above model come out right (i.e., reproduce the measured dielectric
constants of solutions). This model leads to a very simple equation for the dielectric
constant of a solution:
23
This value (6) is the dielectric constant of water under conditions of dielectric saturation. The ion’s field
not only stops the water orienting under the influence of the ac field exerted on the solution, it also breaks
up the associated water structure (which made the dielectric constant of water so huge compared with that
of other liquids). The 6 represents the counter field offered by the distortion of the positions of the nuclei
in H and O and of the electron shells of these atoms.