Page 161 - MODERN ELECTROCHEMISTRY
P. 161
ION–SOLVENT INTERACTIONS 101
Taking as an arbitrary zero in this equation permits the definition of the
relative heat for the heat of solvation of positive ions
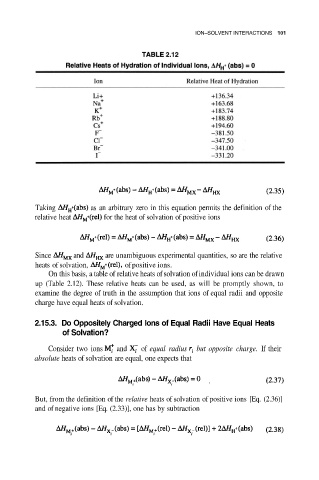
Since and are unambiguous experimental quantities, so are the relative
heats of solvation, of positive ions.
On this basis, a table of relative heats of solvation of individual ions can be drawn
up (Table 2.12). These relative heats can be used, as will be promptly shown, to
examine the degree of truth in the assumption that ions of equal radii and opposite
charge have equal heats of solvation.
2.15.3. Do Oppositely Charged Ions of Equal Radii Have Equal Heats
of Solvation?
Consider two ions and of equal radius but opposite charge. If their
absolute heats of solvation are equal, one expects that
But, from the definition of the relative heats of solvation of positive ions [Eq. (2.36)]
and of negative ions [Eq. (2.33)], one has by subtraction