Page 170 - MODERN ELECTROCHEMISTRY
P. 170
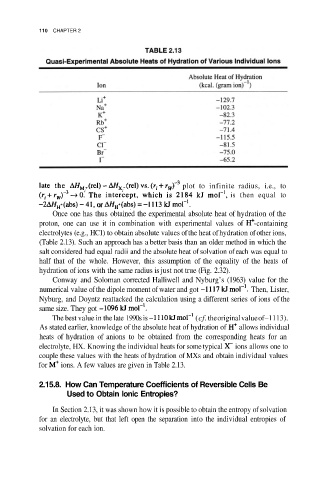
110 CHAPTER 2
late the plot to infinite radius, i.e., to
is then equal to
or
Once one has thus obtained the experimental absolute heat of hydration of the
proton, one can use it in combination with experimental values of -containing
electrolytes (e.g., HC1) to obtain absolute values of the heat of hydration of other ions,
(Table 2.13). Such an approach has a better basis than an older method in which the
salt considered had equal radii and the absolute heat of solvation of each was equal to
half that of the whole. However, this assumption of the equality of the heats of
hydration of ions with the same radius is just not true (Fig. 2.32).
Conway and Soloman corrected Halliwell and Nyburg’s (1963) value for the
numerical value of the dipole moment of water and got Then, Lister,
Nyburg, and Doyntz reattacked the calculation using a different series of ions of the
same size. They got
The best value in the late 1990s is (cf. the original value of –1113).
As stated earlier, knowledge of the absolute heat of hydration of allows individual
heats of hydration of anions to be obtained from the corresponding heats for an
electrolyte, HX. Knowing the individual heats for some typical ions allows one to
couple these values with the heats of hydration of MXs and obtain individual values
for ions. A few values are given in Table 2.13.
2.15.8. How Can Temperature Coefficients of Reversible Cells Be
Used to Obtain Ionic Entropies?
In Section 2.13, it was shown how it is possible to obtain the entropy of solvation
for an electrolyte, but that left open the separation into the individual entropies of
solvation for each ion.