Page 175 - MODERN ELECTROCHEMISTRY
P. 175
ION-SOLVENT INTERACTIONS 115
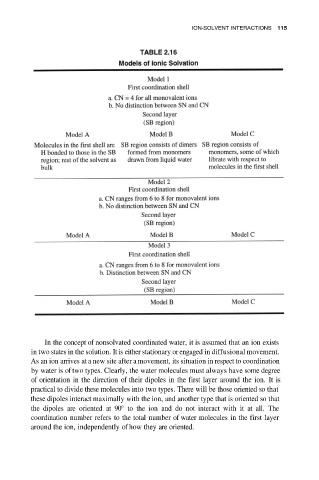
In the concept of nonsolvated coordinated water, it is assumed that an ion exists
in two states in the solution. It is either stationary or engaged in diffusional movement.
As an ion arrives at a new site after a movement, its situation in respect to coordination
by water is of two types. Clearly, the water molecules must always have some degree
of orientation in the direction of their dipoles in the first layer around the ion. It is
practical to divide these molecules into two types. There will be those oriented so that
these dipoles interact maximally with the ion, and another type that is oriented so that
the dipoles are oriented at 90° to the ion and do not interact with it at all. The
coordination number refers to the total number of water molecules in the first layer
around the ion, independently of how they are oriented.