Page 323 - MODERN ELECTROCHEMISTRY
P. 323
ION–ION INTERACTIONS 259
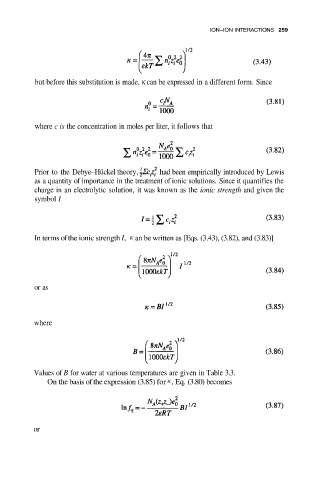
but before this substitution is made, κ can be expressed in a different form. Since
where c is the concentration in moles per liter, it follows that
Prior to the Debye–Hückel theory, had been empirically introduced by Lewis
as a quantity of importance in the treatment of ionic solutions. Since it quantifies the
charge in an electrolytic solution, it was known as the ionic strength and given the
symbol I
In terms ofthe ionic strength I, an be written as [Eqs. (3.43), (3.82), and (3.83)]
or as
where
Values of B for water at various temperatures are given in Table 3.3.
On the basis of the expression (3.85) for , Eq. (3.80) becomes
or