Page 324 - MODERN ELECTROCHEMISTRY
P. 324
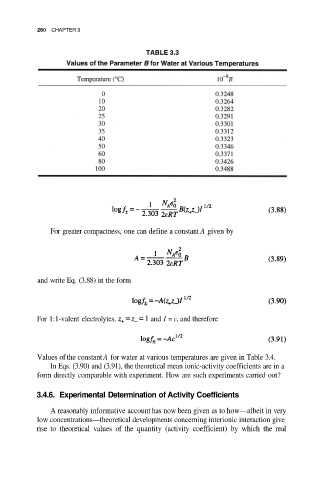
260 CHAPTER 3
For greater compactness, one can define a constant A given by
and write Eq. (3.88) in the form
For 1:1-valent electrolytes, and I = c, and therefore
Values of the constant A for water at various temperatures are given in Table 3.4.
In Eqs. (3.90) and (3.91), the theoretical mean ionic-activity coefficients are in a
form directly comparable with experiment. How are such experiments carried out?
3.4.6. Experimental Determination of Activity Coefficients
A reasonably informative account has now been given as to how—albeit in very
low concentrations—theoretical developments concerning interionic interaction give
rise to theoretical values of the quantity (activity coefficient) by which the real