Page 328 - MODERN ELECTROCHEMISTRY
P. 328
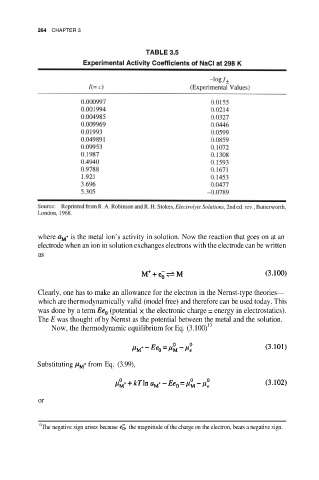
264 CHAPTER 3
where is the metal ion’s activity in solution. Now the reaction that goes on at an
electrode when an ion in solution exchanges electrons with the electrode can be written
as
Clearly, one has to make an allowance for the electron in the Nernst-type theories—
which are thermodynamically valid (model free) and therefore can be used today. This
was done by a term (potential × the electronic charge = energy in electrostatics).
The E was thought of by Nernst as the potential between the metal and the solution.
13
Now, the thermodynamic equilibrium for Eq. (3.100)
Substituting from Eq. (3.99),
or
13
The negative sign arises because the magnitude of the charge on the electron, bears a negative sign.