Page 331 - MODERN ELECTROCHEMISTRY
P. 331
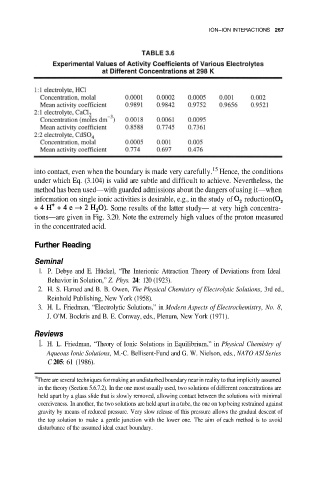
ION–ION INTERACTIONS 267
15
into contact, even when the boundary is made very carefully. Hence, the conditions
under which Eq. (3.104) is valid are subtle and difficult to achieve. Nevertheless, the
method has been used—with guarded admissions about the dangers of using it—when
information on single ionic activities is desirable, e.g., in the study of reduction
Some results of the latter study— at very high concentra-
tions—are given in Fig. 3.20. Note the extremely high values of the proton measured
in the concentrated acid.
Further Reading
Seminal
1. P. Debye and E. Hückel, “The Interionic Attraction Theory of Deviations from Ideal
Behavior in Solution,” Z. Phys. 24: 120 (1923).
2. H. S. Harned and B. B. Owen, The Physical Chemistry of Electrolytic Solutions, 3rd ed.,
Reinhold Publishing, New York (1958).
3. H. L. Friedman, “Electrolytic Solutions,” in Modern Aspects of Electrochemistry, No. 8,
J. O’M. Bockris and B. E. Conway, eds., Plenum, New York (1971).
Reviews
1. H. L. Friedman, “Theory of Ionic Solutions in Equilibrium,” in Physical Chemistry of
Aqueous Ionic Solutions, M.-C. Bellisent-Fund and G. W. Nielson, eds., NATO ASI Series
C 205: 61 (1986).
15
There are several techniques for making an undisturbed boundary near in reality to that implicitly assumed
in the theory (Section 5.6.7.2). In the one most usually used, two solutions of different concentrations are
held apart by a glass slide that is slowly removed, allowing contact between the solutions with minimal
coerciveness. In another, the two solutions are held apart in a tube, the one on top being restrained against
gravity by means of reduced pressure. Very slow release of this pressure allows the gradual descent of
the top solution to make a gentle junction with the lower one. The aim of each method is to avoid
disturbance of the assumed ideal exact boundary.