Page 336 - MODERN ELECTROCHEMISTRY
P. 336
272 CHAPTER 3
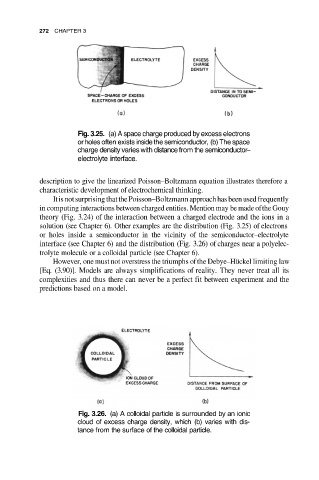
Fig. 3.25. (a) A space charge produced by excess electrons
or holes often exists inside the semiconductor, (b) The space
charge density varies with distance from the semiconductor–
electrolyte interface.
description to give the linearized Poisson–Boltzmann equation illustrates therefore a
characteristic development of electrochemical thinking.
It is not surprising that the Poisson–Boltzmann approach has been used frequently
in computing interactions between charged entities. Mention may be made of the Gouy
theory (Fig. 3.24) of the interaction between a charged electrode and the ions in a
solution (see Chapter 6). Other examples are the distribution (Fig. 3.25) of electrons
or holes inside a semiconductor in the vicinity of the semiconductor–electrolyte
interface (see Chapter 6) and the distribution (Fig. 3.26) of charges near a polyelec-
trolyte molecule or a colloidal particle (see Chapter 6).
However, one must not overstress the triumphs of the Debye–Hückel limiting law
[Eq. (3.90)]. Models are always simplifications of reality. They never treat all its
complexities and thus there can never be a perfect fit between experiment and the
predictions based on a model.
Fig. 3.26. (a) A colloidal particle is surrounded by an ionic
cloud of excess charge density, which (b) varies with dis-
tance from the surface of the colloidal particle.