Page 337 - MODERN ELECTROCHEMISTRY
P. 337
ION–ION INTERACTIONS 273
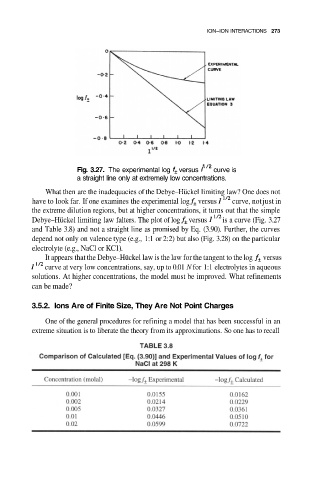
Fig. 3.27. The experimental log versus curve is
a straight line only at extremely low concentrations.
What then are the inadequacies of the Debye–Hückel limiting law? One does not
have to look far. If one examines the experimental log versus curve, not just in
the extreme dilution regions, but at higher concentrations, it turns out that the simple
Debye–Hückel limiting law falters. The plot of log versus is a curve (Fig. 3.27
and Table 3.8) and not a straight line as promised by Eq. (3.90). Further, the curves
depend not only on valence type (e.g., 1:1 or 2:2) but also (Fig. 3.28) on the particular
electrolyte (e.g., NaCl or KC1).
It appears that the Debye–Hückel law is the law for the tangent to the log versus
curve at very low concentrations, say, up to 0.01 N for 1:1 electrolytes in aqueous
solutions. At higher concentrations, the model must be improved. What refinements
can be made?
3.5.2. Ions Are of Finite Size, They Are Not Point Charges
One of the general procedures for refining a model that has been successful in an
extreme situation is to liberate the theory from its approximations. So one has to recall