Page 341 - MODERN ELECTROCHEMISTRY
P. 341
ION–ION INTERACTIONS 277
As a first step, one can use for the lower limit of the integration a distance
parameter that is greater than zero. Then one can go through the mathematics and later
worry about the physical implications of the ion size parameter. Let this procedure be
adopted and symbol a be used for the ion size parameter.
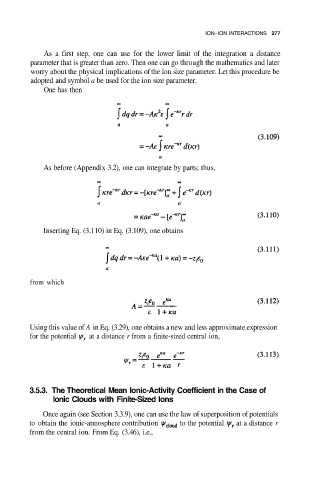
One has then
As before (Appendix 3.2), one can integrate by parts; thus,
Inserting Eq. (3.110) in Eq. (3.109), one obtains
from which
Using this value of A in Eq. (3.29), one obtains a new and less approximate expression
for the potential at a distance r from a finite-sized central ion,
3.5.3. The Theoretical Mean Ionic-Activity Coefficient in the Case of
Ionic Clouds with Finite-Sized Ions
Once again (see Section 3.3.9), one can use the law of superposition of potentials
to obtain the ionic-atmosphere contribution to the potential at a distance r
from the central ion. From Eq. (3.46), i.e.,